An important parameter in the operation of a dry scrubbing system is the amount of alkaline material feed into the system. The amount of sorbent required is a function of the following:
1. The type of sorbent used
2. The inlet and outlet acid gas levels (the outlet level is determined by removal requirements)
3. The effectiveness of the dry scrubbing system design
The amount of sorbent added is usually reported on a molar basis as the stoichiometric ratio of sorbent to acid gases.
Although the sorbents are either calcium- or sodium-based solids, the exact chemical reaction that occurs depends on the type of sorbent used and the injection point in the process. Presently the most widely used dry scrubbing system is the calcium-based hydrated lime [Ca(OH)2]. A slurry of hydrated lime and water is injected into the spray dryer and reacts with the acid gases in a simplified manner as follows:
Ca(OH)2 + SO2 → CaSO3(s) + H2O
Ca(OH)2 + 2HCl → CaCl2(s) + 2H2O
As you can see from the above reactions, one mole of calcium hydroxide [Ca(OH)2] will neutralize one mole of SO2, whereas one mole of calcium hydroxide will neutralize two moles of HCl.
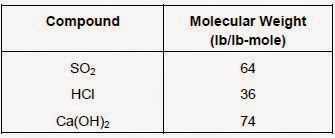
To compute the pounds of calcium hydroxide required to neutralize a given weight of SO2 or HCl, the molecular weight of each component must be utilized. For example, the molecular weights of SO2, HCl, and Ca(OH)2 are as follows:
Therefore, one pound of calcium hydroxide can neutralize 0.86 pounds of SO2 (64 divided by 74) or 0.97 pounds of HCl (36 times 2 divided by 74).
In computing the stoichiometric ratio of a system, all the acid compounds in the waste stream need to be accounted for. Also, the above equations are for the stoichiometric quantities of sorbent. The actual use of sorbent will be above these quantities because of normal inefficiencies in operation; contact of sorbent and acid gases is never ideal and distribution of acid gases in the flue gas is often not uniform (especially in incineration systems). The actual stoichiometric ratios can range from as low as 1.5 to 4.0 dependent on system design and required removal efficiencies.
Similar type reactions occur with sodium-based compounds. For semi-dry systems using caustic soda (NaOH) the following simplified reactions can be written:
SO2 + 1/2 O2 + 2NaOH → Na2SO4 + H2O
HCl + NaOH → NaCl + H2O
Also, sorbents react with different acids at different rates. For example, sorbents react with chlorides at a faster rate than with SO2. Therefore, in waste streams that have both SO2 and HCl, the HCl is removed at a higher rate than the SO2.















0 comments:
Post a Comment